Titration Raw Data Table . — this screencast teaches how to create data tables and do calculations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. formulas for calculating titration data, ph vs. what is a titration? the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). A titration is an analytical procedure used to determine the accurate concentration of a sample by. The following examples work through these kinds of titration calculations: Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume.
from www.chegg.com
formulas for calculating titration data, ph vs. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. A titration is an analytical procedure used to determine the accurate concentration of a sample by. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. what is a titration? — this screencast teaches how to create data tables and do calculations. The following examples work through these kinds of titration calculations: Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume.
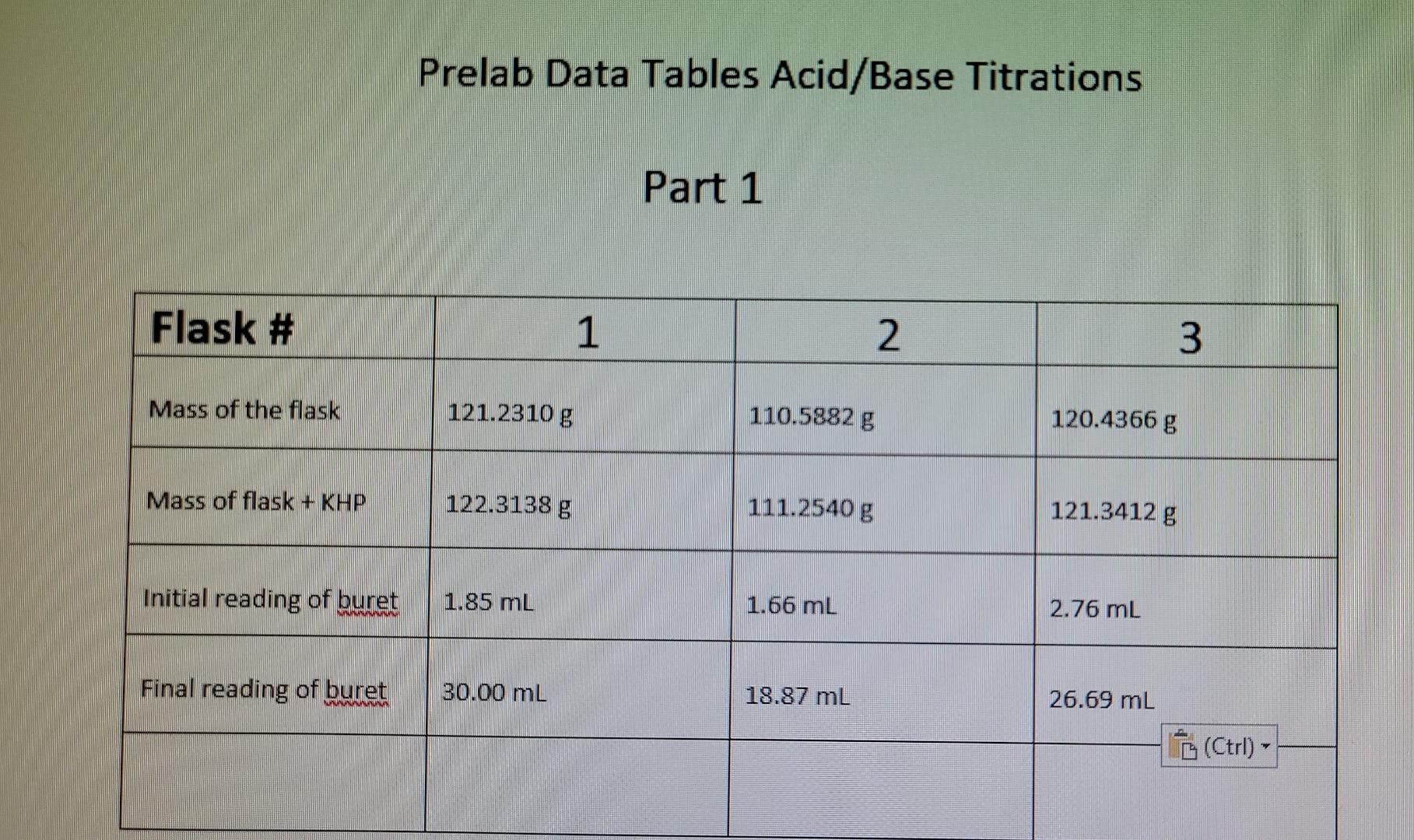
Prelab Data Tables Acid/Base Titrations Part 1 Flask
Titration Raw Data Table The following examples work through these kinds of titration calculations: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. formulas for calculating titration data, ph vs. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. A titration is an analytical procedure used to determine the accurate concentration of a sample by. The following examples work through these kinds of titration calculations: what is a titration? Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. — this screencast teaches how to create data tables and do calculations.
From www.chegg.com
Solved Data Tables Data Table 1 Titration of Calcium Titration Raw Data Table The following examples work through these kinds of titration calculations: Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph. Titration Raw Data Table.
From www.researchgate.net
Table of test results for potentiometric titration analysis. Download Titration Raw Data Table A titration is an analytical procedure used to determine the accurate concentration of a sample by. Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. formulas for calculating titration data, ph vs. At the equivalence point in a neutralization, the moles of acid are equal. Titration Raw Data Table.
From www.chegg.com
Table 1. Raw and Processed Data for the Titration of Titration Raw Data Table The following examples work through these kinds of titration calculations: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is an analytical procedure used to determine the accurate concentration of a sample by. the process of adding the standard solution to the solution of unknown concentration until the. Titration Raw Data Table.
From www.researchgate.net
Screening results of various titrations Download Table Titration Raw Data Table what is a titration? At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — this screencast teaches how to create data tables and do calculations. Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. The. Titration Raw Data Table.
From www.researchgate.net
(A) Raw data for the titration of 0.675 mM DCF with 0.045 mm BSA at 25 Titration Raw Data Table Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. formulas for calculating titration data, ph vs. what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. At the equivalence point in a neutralization, the. Titration Raw Data Table.
From www.chegg.com
Solved 3. Tabulate the raw data for the titration of the Titration Raw Data Table The following examples work through these kinds of titration calculations: what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. — this screencast teaches how to. Titration Raw Data Table.
From www.chegg.com
Prelab Data Tables Acid/Base Titrations Part 1 Flask Titration Raw Data Table A titration is an analytical procedure used to determine the accurate concentration of a sample by. formulas for calculating titration data, ph vs. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. what is a titration? the process of adding the standard solution to the solution of unknown. Titration Raw Data Table.
From www.coursehero.com
[Solved] The table given shows data from the titration of four 10.0 mL Titration Raw Data Table formulas for calculating titration data, ph vs. what is a titration? the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. The following examples work through these kinds of titration calculations: titrations where the analyte is a weak base or acid will need equilibrium /. Titration Raw Data Table.
From www.researchgate.net
ITC titration (raw data above and corresponding plots below) Panel A Titration Raw Data Table what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. The following examples work through these kinds of titration calculations: Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. At the equivalence point in a. Titration Raw Data Table.
From www.researchgate.net
Isothermal calorimetry titration raw data (upper) and corresponding Titration Raw Data Table what is a titration? Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. titrations where the analyte is a weak base or acid will need equilibrium. Titration Raw Data Table.
From www.researchgate.net
Raw data for the titration of (A) 0.1 mM EGCG, (B) 0.1 mM GCG, (C) 0.1 Titration Raw Data Table The following examples work through these kinds of titration calculations: — this screencast teaches how to create data tables and do calculations. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid. Titration Raw Data Table.
From www.youtube.com
Skill How do we draw a raw data table? (Inquiry 2) YouTube Titration Raw Data Table The following examples work through these kinds of titration calculations: formulas for calculating titration data, ph vs. the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two. Titration Raw Data Table.
From www.chegg.com
Solved The following table shows the data for the titration Titration Raw Data Table titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). what is a titration? At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration Raw Data Table.
From dxowzxmzk.blob.core.windows.net
Titration Table Example at Richard Sherman blog Titration Raw Data Table At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the process of adding the standard solution to the solution of unknown concentration until the reaction is just complete is called. titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all. Titration Raw Data Table.
From www.instructables.com
How to Create and Format a Titration Curve in Excel Instructables Titration Raw Data Table what is a titration? titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which is a strong acid or base). At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration Raw Data Table.
From studylib.net
CDCP 02.28.11 Titration Excel Practice Example Titration Raw Data Table what is a titration? At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. formulas for calculating titration data, ph vs. Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. titrations where the analyte is. Titration Raw Data Table.
From www.researchgate.net
(A) Raw data for the titration of 1 mM CFT with 0.045 mm BSA at 25 °C Titration Raw Data Table — this screencast teaches how to create data tables and do calculations. The following examples work through these kinds of titration calculations: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial. Titration Raw Data Table.
From mungfali.com
Titration Lab Diagram Titration Raw Data Table formulas for calculating titration data, ph vs. Raw and processed data for the standardization of naoh portion of the titration of vinegar experiment trial one trial two trial three volume. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. what is a titration? A titration is an analytical procedure. Titration Raw Data Table.